IAP-25-024
Environmental and ‘One Health’ ecology of clinically pathogenic fungi
The evolution and environmental emergence of fungal pathogens over the last decade is now considered a globally significant health issue due to their resistance to the most common anti-fungal drugs.
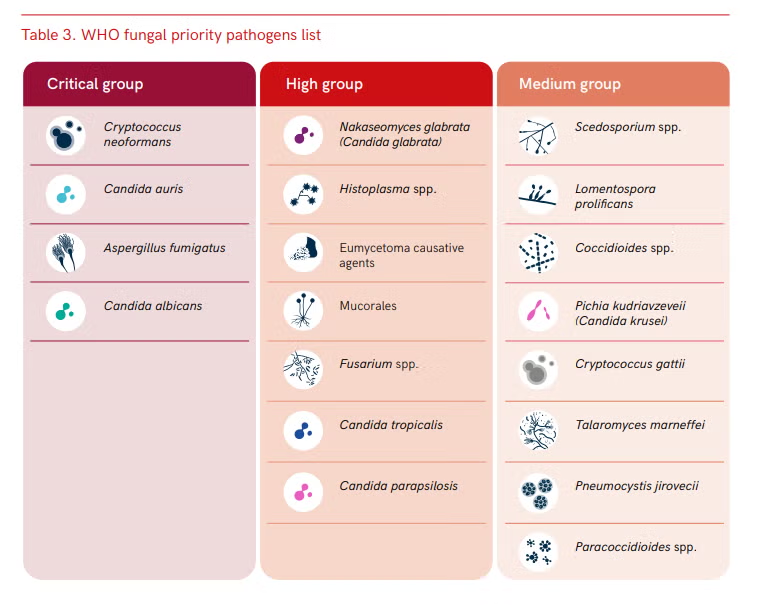
In 2022, the World Health Organisation (WHO) classified five species of Candida as fungal pathogens of critical concern on its “Fungal Pathogen Priority List” which was compiled to guide research and public health action. Pathogenic Candida species cause candidiasis, which can range from life-threatening invasive diseases to superficial conditions such as oral thrush. Although Candida albicans is the most common aetiological agent of candidiasis, there has been an increase in cases of candidiasis caused by a range of other Candida species such as C. tropicalis, C. glabrata, and more recently C. auris. Importantly, however, we know almost nothing about the environmental survival and transfer pathways of pathogenic species of Candida in natural environments. Therefore, understanding the environmental phase of the Candida lifecycle and the transmission pathways associated with it, are critical for developing effective surveillance methods. Similarly, the design of potential epidemiological interventions for reducing the public health risk of emerging anti-fungal drug resistance must be informed by an improved knowledge of the survival and persistence of Candida in the environment.
Globally, candidiasis is the third most common healthcare-associated infection with invasive candidiasis responsible for about 20% of infections in intensive care units worldwide. While there are millions of new cases of candidiasis of the mucosa each year, the annual global incidence of life-threatening invasive candidiasis is approximately 750,000 and is associated with a high mortality rate, of up to 60%, particularly in critically ill patients and those with compromised immunity. Multiple species of Candida are released in hospital wastewater effluent and subsequently discharged into receiving waters from WWTPs. However, the ability of these pathogens to then survive and evolve in the environment has never before been studied.
The aim of this studentship is to, (i) undertake a programme of environmental surveillance for pathogenic species of Candida in the environment, including in wild animals, and (ii) quantify the emerging anti-fungal drug-resistance profiles and environmental adaptation of pathogenic Candida.
There is now, more than ever, an urgent need to identify and quantify the environmental reservoirs of pathogenic Candida and fully characterise their emerging drug-resistance profiles and adaptation to environmental parameters under a changing climate. Therefore, the objectives of this project are to: (i) determine the environmental distribution and dissemination of pathogenic species of Candida within a catchment to coast continuum; (ii) characterise anti-fungal drug resistance profiles of environmental strains of Candida; and (iii) quantify the survival and persistence of pathogenic species of Candida under current environmental conditions and manipulated climate change scenarios. This project will be the first to undertake such experiments and will add to the growing body of evidence for agricultural fungicides/antifungals in the environment as drivers for the evolution of human fungal pathogens. This will lead to the student developing a unique set of research skills at the interface of environmental science and public health.

Click on an image to expand
Methodology
By using an interdisciplinary approach, this project will tackle an emerging and globally significant issue and generate a truly novel dataset and collection of environmental isolates of fungal pathogens. By utilising methods from environmental science, microbiology, and molecular biology the student will develop a range of specific research skills that will be highly transferable beyond this project.
The student will undertake an intensive field monitoring programme in central Scotland, which will be complemented by a comprehensive series of field-relevant controlled laboratory mesocosm experiments to investigate persistence profiles of environmental strains of Candida, under a range of manipulated variables, e.g., elevated temperature, UV irradiance. By using this combination of lab and field-based experiments with both culture-based and molecular approaches, the student will be able to quantify: (1) the spatial and temporal environmental distribution of pathogenic species of Candida in a wide range of natural and agricultural settings (including, soil, water, wastewater/sewage, livestock and wild animals). Effective surveillance is not possible however, without specific detection methods, and most of the currently available tools have primarily focused on the detection of Candida in clinical samples and nosocomial settings rather than complex environmental samples. Therefore, the student will (2) optimise the specificity of culture-dependent and molecular methods to detect pathogenic Candida in environmental samples that may contain relatively high levels of prokaryotes and other eukaryotes. The student will use their environmental isolates of Candida for further characterisation to (3) quantify their tolerance of environmental stresses such as exposure to antifungals, changes in salinity, temperature extremes, and UV irradiance.
To more fully understand the potential risk to public health, all environmental isolates of Candida will subsequently be: (i) screened for resistance to a range of anti-fungal drugs; (ii) assessed for thermotolerance, i.e., are they capable of infecting humans; and (iii) quantified for levels of virulence in a Galleria model of infection, i.e., are they still pathogenic.
Project Timeline
Year 1
Phase 1: “Environmental surveillance of pathogenic Candida.” Following a critical review of the literature (months: 0-4), the student will undertake an intensive field monitoring programme of terrestrial, freshwater, and coastal environments (including WWTP discharge points) to identify and isolate target species of Candida. This phase of the project will also include the optimisation of current culture-based and molecular detection methods for Candida species. Additional measurements will be taken at each sampling location to better understand the environmental characteristics of potential Candida niches or reservoirs in the environment. (Months: 4-20).
Year 2
Phase 2: “Screening environmental isolates of Candida.” Following positive identification through PCR-sequencing, and MALDI-TOF, all environmental Candida isolates will be screened for drug resistance to azoles, echinocandins, and polyenes using minimum inhibitory concentration assays. Isolates will be introduced into a Galleria mellonella infectivity model to determine whether they have retained virulence, and each isolate will be assessed for thermotolerance to quantify environmental adaptation, and tolerance to mammalian thermal barriers. (Months: 8-20).
Year 3
Phase 3: “Persistence profiles under current and future environmental conditions”. A comprehensive series of field-relevant controlled laboratory mesocosm experiments will investigate the effect of environmental factors (changes in salinity, temperature extremes, and UV irradiance) on the survival dynamics and die-off kinetics of target species of Candida. (Months: 18-36)
Year 3.5
Writing the thesis and preparing papers for publication
Training
& Skills
By using an interdisciplinary field and lab approach, this project will tackle an emerging and globally significant issue. By utilising methods from environmental science, microbiology, and molecular biology the student will develop a range of specific research skills that will be highly transferable beyond this project. The student will benefit from the ongoing collaboration between our research groups at Stirling, Glasgow and GCU, and by carrying out lab work at all three institutes will benefit from the broad interdisciplinary expertise of the three supervisors. The student will gain a solid foundation in research methods and skills in both the lab and the field, which will lead to significant expertise in this important area of environmental science.
References & further reading
Metcalf R, Akinbobola A, Woodford L, Quilliam RS. (2025). Thermotolerance, virulence, and drug resistance of human pathogenic Candida species colonising plastic pollution in aquatic ecosystems. Environmental Science and Pollution Research 32, 14993
Akinbobola A, Kean R, Quilliam RS. (2024). Plastic pollution as a novel reservoir for the environmental survival of the drug-resistant fungal pathogen Candida auris. Marine Pollution Bulletin 198, 115841
Akinbobola A, Hanifi SMA, Kean R, Quilliam RS. (2023). Environmental reservoirs of the multi-drug-resistant pathogenic yeast Candida auris. PLOS Pathogens 19, e1011268.

